Magyarország
Choose your country or language
REA JET international
- Solutions
- Products
- Events
- Service
- Career
- Company
- Contact
- News
- Language - Magyarország
- Data Protection Statement
- Imprint
- Contact
- FC
- store
- Login
Choose your country or language
REA JET international
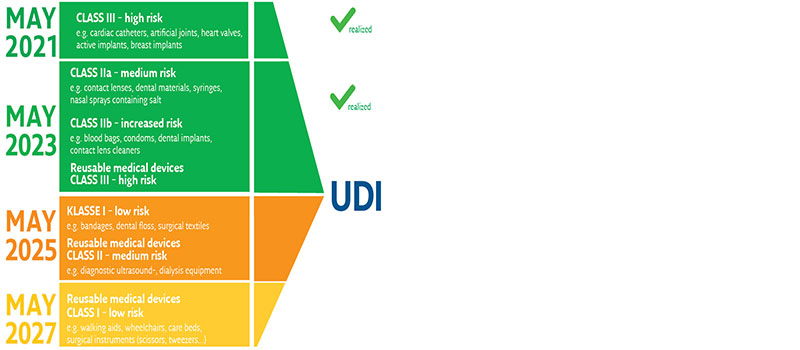
The EU Medical Device Regulation (MDR) 2017/745 of the European Union obliges producers, distributors, importers and EU representatives of medical devices or their packaging to clearly mark their products as of May 26, 2021. Medical devices will thus be completely traceable from the manufacturer to the user and guarantee the patient safety required by the EU.
The standards and regulations passed in accordance with the Medical Device Regulation are equally binding for all players. To this end, the product identification required for the medical device is issued by one of the four allocation authorities - GS1, HIBCC, ICCBBA and Informationsstelle für Arzneispezialitäten.
From May 2022, all medical devices will be stored bit-by-bit in the EU-wide EUDAMED database with their master data and the "Unique Device Identification" (UDI).
The product identification consists of two components:
Medical devices are categorized and classified into risk classes, which are defined by the intended purpose in terms of the place and duration of use of the product.
There are e.g. products for single use or reusable medical devices, implants or others like software and devices.
The resulting risk classes then also specify the implementation deadlines in legislation.
The product identification must be applied either directly on the product, on primary packaging or on all higher packaging levels. According to the Medical Device Regulation the direct marking must be applied by ink, laser or label.
In addition, the code must be easily accessible and readable both in the warehouse and when the product is in use - and this throughout the entire life of the product.
The marking is always in human readable text and as a machine readable code. 1D barcodes or 2D matrix codes can be marked on the medical devices, depending on the available area for direct marking.
Depending on the medical device and its properties, there is a very large variety of possible markings. To ensure that the codes are read error-free with a high first-pass reading rate and that the standards of the Medical Device Regulation are met, the code verification systems REA VERIFIER are used.
REA JET HR Coding and Marking Systems based on HP cartridges are ideal for high resolution 2D codes, brand logos or other high-quality markings – and at high speeds. Products and packaging can be marked with a print height of 12.7 mm per print head - several print heads can be cascaded for greater print heights. High first-pass reading rates are therefore no problem at the point of sale.

REA JET CL Laser Systems mark packaging made of organic materials durably, highly precisely and individually. The laser systems are consumable-free. High first-pass reading rates are also guaranteed here.

REA JET FL Laser System mark plastic packaging contact-free, precisely and individually. Fiber laser marking is permanently durable and ideal for harder metals such as stainless steel and titanium as well as for plastics but also for thin foils. The laser systems are consumable-free. Here, too, high first-pass reading rates at the point of sale are guaranteed.

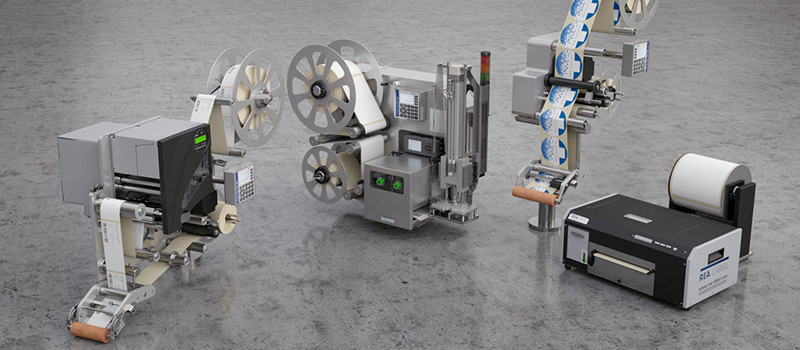
Wherever direct marking with Inkjet Printers or Laser Marking Systems is out of the question, labeling solutions are the obvious choice.
REA LABEL offers suitable solutions for this purpose, from simple tabletop label printers to complex labeling systems for all applications.
Code verification systems from REA VERIFIER are used to ensure that markings and codes are error-free and comply with international standard specifications. In this way, each marking receives an acceptance test report.
REA offers a complete product portfolio and code know-how for the implementation of this task. Please contact us.