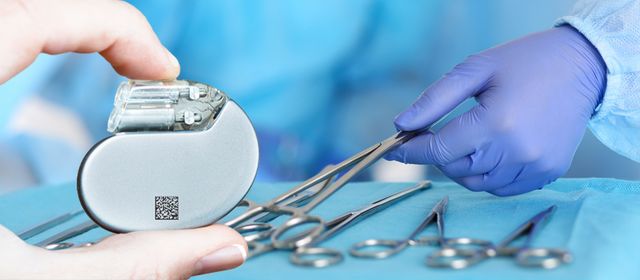
UDI marking is coming soon – REA offers the turn-key solution
Categorie:
Applicazioni
|Tag:
LABEL,
VERIFIER,
HR,
CL,
Farmaceutico
Nearing the finishing line for pharmaceutical serialization
Categorie:
News
|Tag:
VERIFIER,
HR,
CL,
Farmaceutico
REA at the Achema: Final burst for the Pharma serialization
Categorie:
News
|Tag:
Fiera,
Alimentari,
Farmaceutico