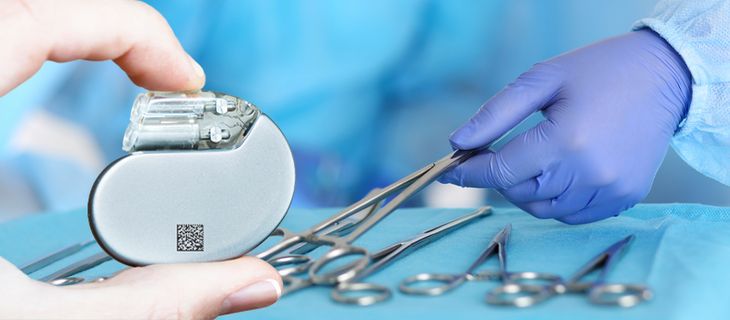
UDI marking is coming soon – REA offers the turn-key solution
Is this the right heart valve? Where does the hearing aid come from? Are the surgical instruments that were prepared and registered before the surgery complete again after the operation? It is often difficult to clarify these questions. There is no uniform system in place so far for the identification and tracing of so-called medical devices.
This is to change: By the end of May 2025, all medical devices sold in the EU, Iceland, Liechtenstein, Norway and Switzerland must be clearly identifiable with a sequence of numbers under the "Medical Device Regulation" (MDR). For products with a high risk (e.g. artificial joints), this will already be the case starting from May 2021. Products with a medium risk (e.g. hearing aids) must be marked from May 2023 and medical products with a low risk (e.g. glasses) from the end of May 2025. The overall goal of all efforts is to increase patient safety.
Product identification with UDI
UDI (Unique Device Identification) is the name of the coding that the European Union is currently establishing - coordinated with the USA and recognized by their health authority (FDA). The core of the system is a numerical code. It consists of a fixed front part (DI - Device Identifier), which is assigned to the manufacturers by four allocation authorities, and a variable rear part (PI - Production Identifier), which the manufacturers themselves define according to the specifications for the respective medical device. It is used for individual identification and traceability of each part.
UDI is designed to provide information about the origin and properties of a medical device available throughout its entire life cycle for possible problems that may arise. To this end, the central database Eudamed (European Databank on Medical Devices) is to be set up from May 2022. Here, every manufacturer must register every medical device with its UDI before importers or distributors can check and supplement the data, which is what pharmacies, hospitals or medical practices then do. This way, Eudamed completely documents the development of each product.
In plain text and as machine-readable code
Accordingly, manufacturers have to mark each medical device or its packaging with UDI - well positioned and in at least two forms: as plain text and machine-readable 1D barcode or 2D Data Matrix code. This can be done with ink, laser or a label.
This is where REA comes into play. As a full-range supplier, our product lines REA JET, REA LABEL and REA VERIFIER offer effective, economical and reliable systems for marking, labeling and code verification from one source. All REA systems are industry 4.0 compatible - they can be smoothly integrated into existing systems and production environments.
- The High Resolution Inkjet Printers REA JET HR print with HP technology and extremely high resolution at high product speeds.
- The REA JET GK 2.0 uses piezo technology and solvent-free inks to print onto absorbent surfaces such as cardboard boxes with high print quality and edge sharpness.
- The REA JET SC 2.0 small character inkjet printer uses continuous inkjet technology to print texts, logos and codes on smooth surfaces (films, plastics or metals).
- For permanent direct marking on plastic or metal, REA JET Laser Systems are the right choice.
- Adhesive labels are dispensed by the REA LABEL Labeling Systems onto boxes and cartons of all packaging sizes to any position.
- The Code Verification Systems from REA VERIFIER provide legal security: they ensure that code marking is correct and error-free, that the code quality complies with international standards and requirements and that the codes can be read by machine, at the highest first-pass reading rate.
UDI presents manufacturers of medical devices with new challenges - the REA systems stand for reliable, high-quality and permanent marking and code verification.
We would be pleased to support you in the implementation of the UDI guideline.
- We have prepared an easy to understand and helpful UDI brochure, which you can download below
- REA advises and trains you in UDI
- Fascilitation of competent partners for data management
- Qualification ans use of marking technologies
- Qualification and use of verification systems for compliance with code quality specifications