Unik identifiering av produkter - Märkning av medicintekniska produkter
Från och med den 26 maj 2021 kommer EU:s förordning om medicintekniska produkter (MDR) 2017/745 att kräva att tillverkare, distributörer, importörer och auktoriserade EU-representanter av medicintekniska produkter eller deras förpackningar är tydligt märkta. Inom medicinteknik kommer medicintekniska produkter därför att vara fullt spårbara från tillverkaren till användaren och garantera den patientsäkerhet som EU kräver.

Unik identifiering av medicintekniska produkter (UDI)
Från och med den 26 maj 2021 kommer EU:s förordning om medicintekniska produkter (MDR) 2017/745 att kräva att tillverkare, distributörer, importörer och auktoriserade EU-representanter av medicintekniska produkter eller deras förpackningar är tydligt märkta. Inom medicinteknik kommer medicintekniska produkter därmed att vara fullt spårbara från tillverkaren till användaren och garantera den patientsäkerhet som EU kräver.
De standarder och föreskrifter som antas i enlighet med förordningen om medicintekniska produkter är lika bindande för alla intressenter. Den produktidentifiering som krävs för den medicintekniska produkten tilldelas av ett av de fyra tilldelningsorganen - GS1, HIBCC, ICCBBA och Information Centre for Proprietary Medicinal Products.
Från och med maj 2022 kommer alla medicintekniska produkter att lagras med sina masterdata och den unika produktidentifieringen (UDI) i den EU-omfattande EUDAMED-databasen.
Produktidentifieringen består av två komponenter
- Device Identifier (DI): en statisk kod med cirka 20 uppgifter för identifiering av tillverkare och produkt
- Production Identifier (PI): variabla data som används för spårbarhet, t.ex. batchnummer, utgångsdatum eller serienummer
Tidsfrister för genomförande
Märkningsskyldighet för riskklasser

Var måste UDI-märkningen appliceras?
Direkt märkning med bläck, laser eller etikett
Produktidentifieringen måste appliceras antingen direkt på produkten, på primärförpackningen eller på alla högre förpackningsnivåer. Enligt förordningen om medicintekniska produkter måste direktmärkning ske med bläck, laser eller etikett. Dessutom måste koden vara lättillgänglig och läsbar både i lagret och när produkten används - och måste förbli läsbar under hela produktens livslängd.
UDI-podcast från Johnerinstitutet
Lyssna på podcasten från Johnerinstitutet om UDI-ämnet med professor D. Christian Johner och Wilfried Weigelt.
Märkningen sker alltid i klartext och som en maskinläsbar kod. Beroende på vilken yta som är tillgänglig för direkt märkning kan 1D-streckkoder eller 2D matriskoder märkas på de medicintekniska produkterna.
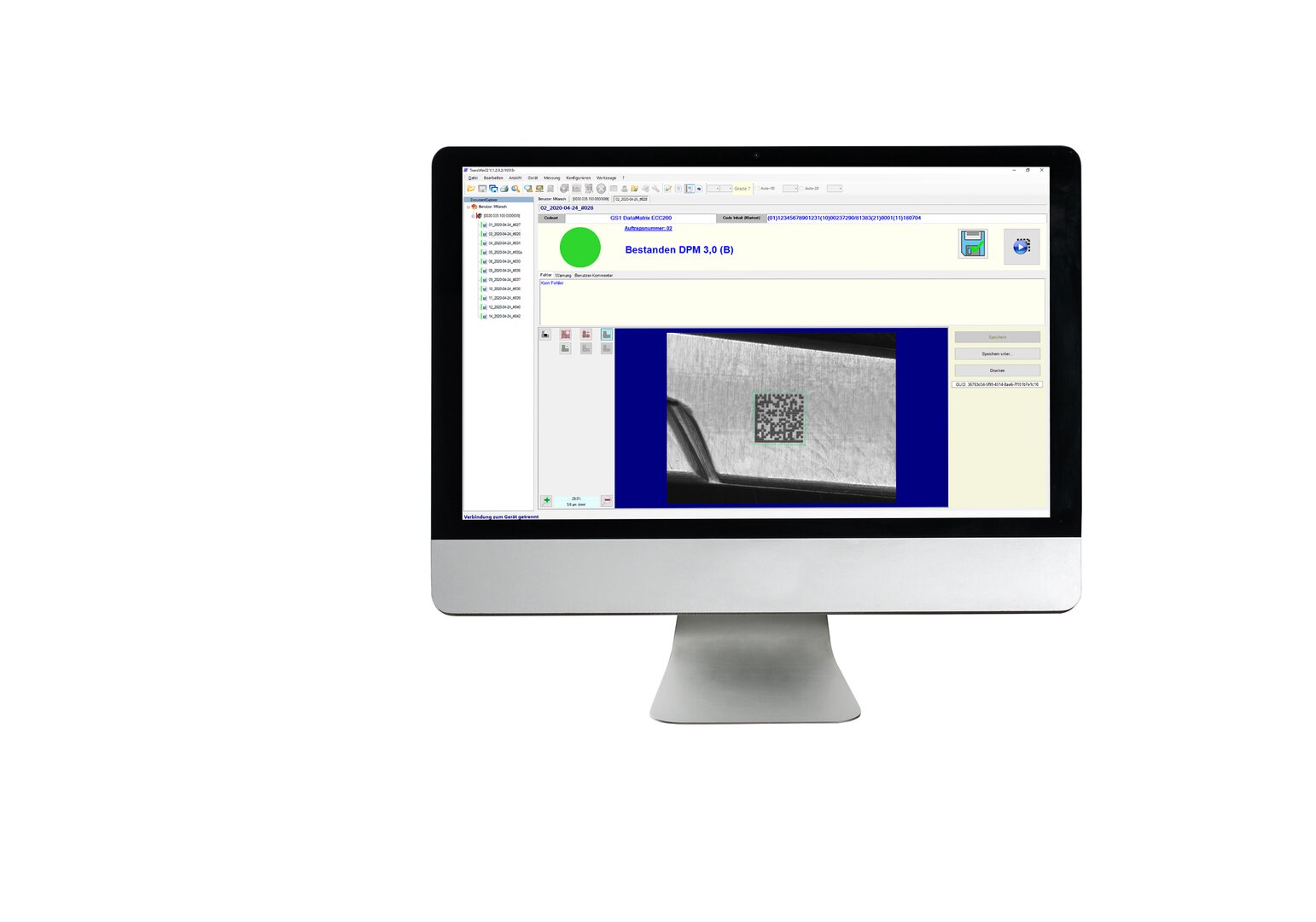
Beroende på den medicintekniska produkten och dess egenskaper finns det ett mycket stort antal olika varianter av märkning. REA VERIFIERs verifieringssystem används för att säkerställa att koderna läses felfritt med ett högt första inläsningstillfälle och att standarderna i förordningen om medicintekniska produkter uppfylls.
Högupplösta bläckstråleskrivare baserade på HP-patroner
REA Kodnings- och märkningssystem baserade på HP-kassetter är idealiska för högupplösta 2D-koder, varumärkeslogotyper eller annan märkning av hög kvalitet - i höga hastigheter. Produkter och förpackningar kan märkas med en skrivhöjd på 12,7 mm per skrivhuvud - flera skrivhuvuden kan kaskadkopplas för större skrivhöjder. Höga första inläsningstillfällen är därför inget problem på försäljningsstället.

REA LASER CL
REA LASER CL lasersystem märker förpackningar av organiska material permanent, mycket exakt och individuellt. Lasersystemen är inget förbrukningsmaterial. Höga första inläsningstillfällen garanteras också här.

REA LASER FL
REA LASER FL lasersystem märker plastförpackningar kontaktfritt, exakt och individuellt. Märkning med fiberlaser är permanent och idealisk för hårdare metaller som rostfritt stål och titan samt för plast och tunna filmer/folier. Lasersystemen är inget förbrukningsmaterial. Även här garanteras höga första inläsningstillfällen vid försäljningsstället.

Teknologi för etikettering
Etiketteringslösningar är idealiska när direkt märkning med bläckstråleskrivare eller lasermärkningssystem inte är ett alternativ.
REA LABEL erbjuder lämpliga lösningar för detta ändamål, från enkla bordsetikettskrivare till komplexa märkningssystem för alla applikationer.

System för kodkontroll
För att säkerställa att märkning och koder är felfria och uppfyller internationella standarder används verifieringssystem från REA VERIFIER. På så sätt får varje märkning en acceptanstestrapport.
REA erbjuder en komplett produktportfölj och kodexpertis för att genomföra denna uppgift. Hör av dig till oss.

REA är din partner
Rådgivning och utbildning
- REA ger råd och utbildar dig i UDI-frågor
- Upphandling av kompetenta partners för datahantering
- Lämplighet och användning av teknologier för kodning och märkning
- Lämplighet och användning av verifieringssystem för överensstämmelse med specifikationer för kodkvalitet