Senaste nytt från världen av system för märkning, etiketterare och kodverifiering
I vårt nyhetsavsnitt kan du ta reda på allt du behöver veta om den senaste utvecklingen, teknologin och trenderna inom industriell märkning och verifiering av koder. Håll dig informerad om innovativa lösningar, bästa praxis och viktig information.
Filtrera map. kategorier

Mässa
Färgad märkning av gummidäck

Mässa
Sofistikerade koncept för industriell märkning och verifiering av koder

Mässa
Högkvalitativ märkning, verifiering av koder och vätning för plåtproduktion

Mässa
Moderna plaster, högkvalitativ och variabel märkning

Mässa
Hållbar eftermontering av tryckpressar för anpassat innehåll
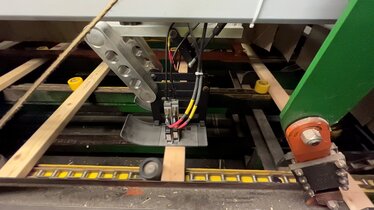
Produkter
Kraftigt stötskydd för REA JET HR: Oöverträffad säkerhet inom träindustrin

News
Kundanpassade etiketteringskoncept för moderna förpackningar

News
Det nya elektriskt styrda spraymärkningsblocket REA JET ST SRP-10

News
Tillförlitlig märkning för värdekedjan för trä

News
Ledningsförändring på REA Elektronik inleder en ny era

News
Kvaliteten först - så måste även märkningen av högkvalitativa farmaceutiska och medicinska produkter se ut

News
Hållbar, kundanpassad, tillförlitlig: märkning av logistik från REA
