Siste nytt fra verden av systemer for merking, etiketteringsmaskiner og kodeverifisering
I nyhetsseksjonen vår kan du finne ut alt du trenger å vite om den nyeste utviklingen, teknologien og trendene innen industriell merking og kodeverifikasjon. Hold deg oppdatert om innovative løsninger, beste praksis og viktig informasjon.
Filtrer etter kategorier

Casestudier
Pålitelig merking for hippe blandingsdrikker

Messe
Farget merking av gummidekk

Messe
Sofistikerte konsepter for industriell merking og kodeverifikasjon

Messe
Merking, kodeverifikasjon og fukting av høy kvalitet for produksjon av metallplater

Messe
Moderne plast, høy kvalitet og variabel merking

Messe
Bærekraftig ettermontering av trykkpresser for tilpasset innhold
Produkter
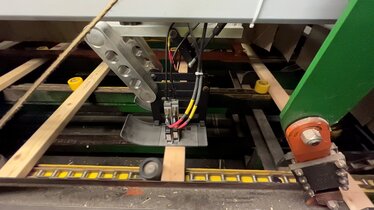
Kraftig slagbeskyttelse for REA JET HR: Uovertruffen sikkerhet i treindustrien

News
Skreddersydde etiketteringskonsepter for moderne emballasje

News
Den nye elektrisk styrte sprayblokken REA JET ST SRP-10

News
Pålitelig merking for treverdikjeden

News
Lederskifte i REA Elektronik innleder en ny æra

News
Kvalitet først - det må merking av farmasøytiske og medisinske produkter av høy kvalitet også være
