Unik utstyrsidentifikasjon - Merking av medisinsk utstyr
Fra 26. mai 2021 vil EUs forordning om medisinsk utstyr (MDR) 2017/745 kreve at produsenter, distributører, importører og EU-autoriserte representanter av medisinsk utstyr eller emballasjen til dette skal være tydelig merket. Medisinsk utstyr vil dermed være fullt sporbart fra produsent til bruker og garantere den pasientsikkerheten som EU krever.

Unik utstyrsidentifikasjon (UDI)
Fra 26. mai 2021 vil EUs forordning om medisinsk utstyr (MDR) 2017/745 kreve at produsenter, distributører, importører og EU-autoriserte representanter for medisinsk utstyr eller emballasjen deres skal være tydelig merket. Medisinsk utstyr vil dermed være fullt sporbart fra produsent til bruker og garantere den pasientsikkerheten som EU krever.
Standardene og forskriftene som er vedtatt i henhold til forordningen om medisinsk utstyr, er like bindende for alle interessenter. Produktidentifikasjonen som kreves for medisinsk utstyr, tildeles av ett av de fire tildelingsorganene - GS1, HIBCC, ICCBBA og Information Centre for Proprietary Medicinal Products.
Fra mai 2022 vil alt medisinsk utstyr bli lagret med sine stamdata og den unike utstyrsidentifikasjonen (UDI) i den EU-omfattende EUDAMED-databasen.
Produktidentifikasjonen består av to komponenter
- Device Identifier (DI): en statisk kode med ca. 20 data for produsent- og produktidentifikasjon
- Production Identifier (PI): variable data som brukes til sporbarhet, for eksempel batchnummer, Utløpsdato eller Serienummer
Tidsfrister for implementering
Merkeplikt for risikoklasser

Hvor må UDI-merkingen påføres?
Direkte merking med blekk, laser eller etikett
Produktidentifikasjonen må påføres enten direkte på produktet, på primæremballasjen eller på alle høyere emballasjenivåer. I henhold til forordningen om medisinsk utstyr må direkte merking påføres med blekk, laser eller etikett. I tillegg må koden være lett tilgjengelig og lesbar både på lageret og når produktet er i bruk - og den må forbli lesbar i hele produktets levetid.
UDI-podcast fra Johner-instituttet
Hør podcasten fra Johner Institute om UDI-temaet med professor D. Christian Johner og Wilfried Weigelt.
Merking skjer alltid i klartekst og som maskinlesbar kode. Avhengig av hvilken overflate som er tilgjengelig for direkte merking, kan 1D-strekkoder eller 2D-matrisekoder merkes på det medisinske utstyret.
Avhengig av det medisinske utstyret og dets egenskaper finnes det et stort utvalg av mulige varianter av merking. REA VERIFIERs verifikasjonssystemer brukes for å sikre at kodene leses feilfritt med høy første lesehastighet, og at standardene i forordningen om medisinsk utstyr overholdes.
Høyoppløselige blekkskrivere basert på HP-kassetter
REA JET HR merkesystemer basert på HP-kassetter er ideelle for høyoppløselige 2D-koder, merkelogoer eller annen merking av høy kvalitet - i høye hastigheter. Produkter og emballasje kan merkes med en Skrivehøyde på 12,7 mm per skrivehode - flere skrivehoder kan kaskadekobles for større skrivehøyder. Høye første lesehastigheter er derfor ikke noe problem på utsalgsstedet.

REA LASER CL
REA LASER CL lasersystemer merker emballasje av organiske materialer permanent, svært presist og individuelt. Lasersystemene er uten forbruksmateriell. Høye første lesehastigheter er også garantert her.

REA LASER FL
REA LASER FL lasersystemer merker plastemballasje berøringsfritt, presist og individuelt. Merking med fiberlaser er permanent og ideell for hardere metaller som rustfritt stål og titan, samt for plast og tynne filmer. Lasersystemene er uten forbruksmateriell. Også her garanteres høye første lesehastigheter på salgsstedet.

Etiketteringsteknologi
Etiketteringsløsninger er tilgjengelige overalt der direkte merking med blekkskrivere eller lasermerkingssystemer ikke er et alternativ.
REA LABEL tilbyr egnede løsninger for dette formålet, fra enkle etikettskrivere for bordbruk til komplekse merkesystemer for alle bruksområder.

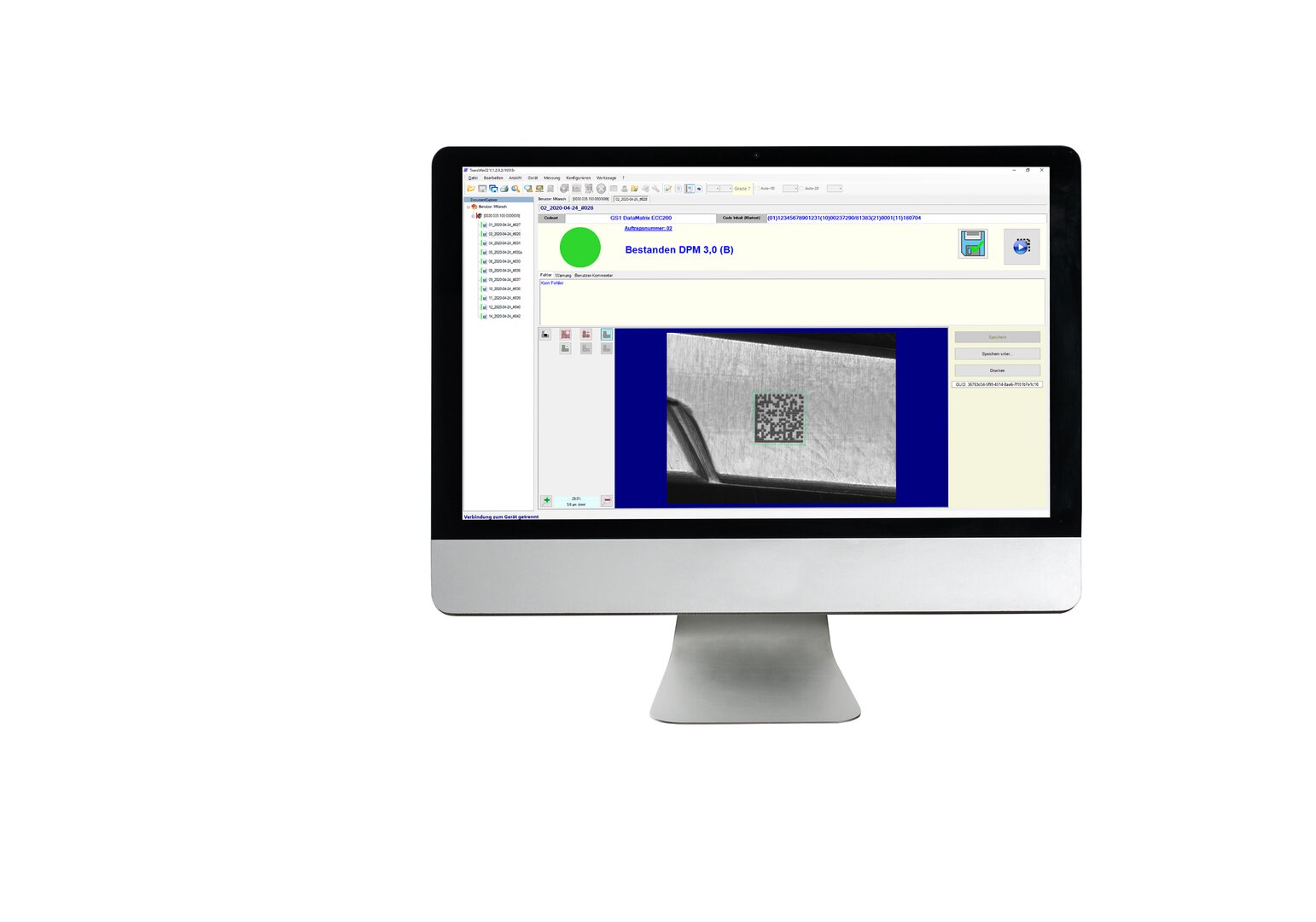
Systemer for kodesjekk
Verifikasjonssystemer fra REA VERIFIER brukes til å sikre at merking og koder er feilfrie og i samsvar med internasjonale standarder. På denne måten får hver merking en akseptansetestrapport.
REA tilbyr en komplett produktportefølje og kodeekspertise for gjennomføring av denne oppgaven. Ta kontakt med oss.

REA er din partner
Rådgivning og opplæring
- REA gir deg råd og opplæring i UDI-spørsmål
- Anskaffelse av kompetente partnere for datahåndtering
- Egnethet og bruk av merketeknologier
- Egnethet og bruk av verifikasjonssystemer for samsvar med kvalitetsspesifikasjoner